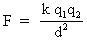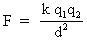Home Page of Peggy E. Schweiger
Electrostatics
- Electrostatics
- the study of electrical charges at rest
A charged object is created by the separation of charges:
- an atom is electrically neutral; it has the same number of protons (positive charges) as it does electrons (negative charges)
- objects are charged by adding or removing electrons
- a positive charge occurs when there are fewer electrons than protons; its classical definition is the charge accumulated by a glass rod rubbed with silk or wool
- a negative charge occurs when there are more electrons than protons; its classical definition is the charge accumulated by a hard, rubber rod rubbed with fur
- Conductor
- a substance that allows electrons to move easily throughout
- Insulator
- a substance that does not allow electrons to move freely; electrons stay in one place
- Semiconductor
- partially conductive, partially insulative
- Electroscope
- an instrument used to detect the presence of an electrostatic charge
- Coulomb ( C )
- the SI unit of charge
1 C = 6.24 x 1018 electrons
There are two kinds of electrical charges, positive (+) and negative (-). Like charges repel and unlike charges attract. Thus, electrical charges exert a force on other electrical charges. This electrostatic force is directly proportional to the product of the charges and inversely proportional to the square of their distance of separation (another inverse square law relationship!)
Methods of charging an object:,/b>
- Conduction
- a charged object touches another object; the amount of charge equally divides between the two objects; the same sign charge is acquired by each object
- Induction
- a charged object is brought near, but not touching, another object; it attracts charges opposite to it and repels charges like it; when a ground is used, the opposite charge is acquired on the other object; it is thus charged without being touched
Coulombís Law describes the electrostatic force between two charged objects.
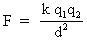
where k is Coulombís constant, or k = 9 x 109 Nm2/C2
q is the magnitude (NO sign) of each charge in coulombs
d is the distance of separation in meters
F is the electrostatic force in Newtons. It is either attractive or repulsive.
Since charges are small, they are usually expressed in non-SI units of microcoulombs, nanocoulombs, or picocoulombs. They must be converted into Coulombs for calculations.
1 mC = 1 x 10-6C
1 nC = 1 x 10-9 C
1 pC = 1 x 10-12C
- Elementary charge (qo)
- the charge of either an electron or a proton. The charge of a proton is equal in magnitude to that of an electron, but it positive.
qo = 1.6 x 10-19 C
Methods for Working Coulomb's Law Problems
- Always remember to convert everything to SI units! A mC is not
an SI unit. It must be converted into Coulombs. Remember to convert distances in centimeters
to meters.
- Forces are vector quantities. We will use Coulomb's law to calculate the magnitude of the
electrostatic force between two charges. When we are calculating magnitude, we will not consider
the signs of the charges.
- Forces are vector quantities. We will use free body diagrams to determine the direction of the
electrostatic force between two charges.

- Determine the magnitude of the electrostatic force exerted by the +4 C charge on the +6 C
charge. It is repulsive or left.
- What is the resultant force on the middle charge?

- Determine the magnitude of the force exerted on the +4 C charge by the +5 C charge. Its
direction is right (repulsive). Determine the magntitude of the force exerted on the +4 C charge by the
+6 C charge. Its direction is left (repulsive). Use positive and negative signs to represent
the directions of the forces. Assign a negative sign to force acting to the
left and a positive sign to the force acting to the right. The resultant force on the +4 C charge
is the sum of these forces.
Electrostatics Sample Problems
Electrostatics Homework