Home Page of Peggy E. Schweiger
Quantum Theory
In 1887, Hertz' experiments confirmed Maxwells' theory of light. Seemingly, his electromagnetic theory of light explained all of optics. Except for two small problems that could not be explained by the wave theory of light. When light is shone on a metal surface, a current is created implying that the light caused electrons to be emitted from the surface. The electron flow depends upon the frequency of the light used. This is called the photoelectric effect. An incandescent light bulb emits a wide spectrum of frequencies. The higher the temperature of the light bulb, the "whiter" it appears. The spectrum of light produced doesn't depend upon the material, but the temperature.
In 1900, Max Planck explained the spectrum of the light produced by an
incandescent body. He proposed that the atoms of the material didn't radiate electromagnetic waves continuously, but only at discrete values. He proposed that energy was quantized.
In 1905, Albert Einstein proposed a revolutionary theory of light, explaining the photoelectric effect. In his theory, light consists of discrete bundles of energy called photons. Einstein won the Nobel Prize for his theory in 1921.
Theories of light:
- Newton's theory - light consists of particles called corpuscles; this theory only explained reflection
- Wave theory of light (Maxwell's theory) - light behaves like a wave; this explained all the properties of light such as reflection, refraction, diffraction, and interference; it did not explain the photoelectric effect or radiation produced by an incandescent light
- Quantum theory (Einstein's theory) - light has a dual nature; when light is transmitted through space or matter, it behaves like a wave; when light is emitted or absorbed, it behaves like a particle called a photon
Maxwell's theory of light as an electromagnetic wave:
- a changing electric field will produce a magnetic field
- a changing magnetic field will produce an electric field
A magnetic field is produced in empty space by a changing electric field.
Maxwell hypothesized that if a changing magnetic field produces an electric
field, the electric field must also be changing. Maxwell found that the net
result of these interacting fields was the production of a wave of
magnetic and electric fields traveling through space at a speed of 3 x 108
m/s. Thus, light is an electromagnetic wave.
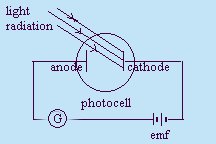
A photocell is used in the experiment. When placed in the dark, the
galvanometer reads zero. When light shines on a metal plate in the
photocell, the galvanometer detects a current. When a variable voltage is
used and the terminals reversed, a point is reached where no current is
detected. This voltage is measures the maximum kinetic energy of the
ejected electrons (or photoelectrons). The current flow does not depend upon the intensity of the light
used, but upon the frequency of the light used.
Maxwell's wave theory predicts that as the intensity of light is increased,
the current flow should increase. The frequency should not affect the maximum
kinetic energy of the photoelectrons. According to this theory, the electric
field of the electromagnetic wave exerts a force on the electrons in the metal
and some are ejected from the surface.
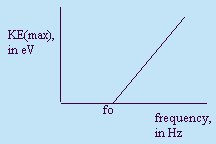
Einstein's photon theory predicts that only the frequency of the light used
affects the maximum kinetic energy of the photoelectrons. As the intensity of
light is increased, no change is seen in the maximum kinetic energy of the
photoelectrons. No photoelectrons are ejected until a minimum value of energy
is reached, no matter how great the intensity. After this point, the maximum
kinetic energy of the photoelectrons increases linearly as the frequency of
light used increases.
- Photon
- the particle nature of light;
discrete bundles of energy; the energy of a photon depends upon the frequency
of light used; a photon has no rest mass
- E = h f
- E = h / l
- momentum of a photon: p = hf/c = h/l
- Threshold frequency (fo)
-
the minimum frequency of light that results in the emission of photoelectrons
- Planck's constant (h)
- h = 6.626 x 10-34J sec
- Work function
- the minimum amount of work
needed to eject an electron from the surface
- E = h fo
- Stopping potential (Vo)
-
equivalent to the maximum kinetic energy of the ejected photoelectrons
(KEmax)
- Photoelectric equation
- KEmax = hf - hfo
- Electron volt (eV)
- voltage in volts is
equivalent to the magnitude of the energy in electron volts. The electron volt
is an easier unit to use than Joules. An electron volt can be easily
converted into Joules.
- 1 eV = 1.6 x 10-19J
Experimental proofs that light behaves like a wave:
- Photoelectric effect
- Compton effect - X-rays that irradiate a substance lose energy when they
strike the substance (the wavelengths of the X-rays that are scattered by
striking the substance are longer, or lower in energy)
- Pair production - a photon disappears in the process of creating electron/
positron pairs; rest mass is created from pure energy according to Einstein's
E = m c2. If an electron collides with a positron, the pair
annihilate each other and their energy appears as that of photons.
The dual nature of light is a very abstract concept that cannot be visualized.
We cannot imagine light as a combination of a wave and a particle. When we do
an experiment, we must think of light as a wave or as a particle -- not as a
combination.
Early atomic models:
- Plum pudding model(approximately 1890's)
- atom was visualized as a homogeneous sphere of positive charge in which
were randomly located tiny negative charges--like plums in a pudding
- Rutherford model (1911)
- Rutherford's gold foil experiment contradicted the plum pudding atomic
model
- Rutherford bombarded a piece of gold foil with a beam of positive charges
called alpha particles.
- the plum pudding model predicted that there should be little alpha particle
deflection
- experimental results indicated that the majority of the alpha particles
passed through the foil unaffected and some of the alpha particles were deflected
at very great angles
- Rutherford concluded that the majority of the atom was empty space
- He also concluded that the alpha particle deflection could only be
explained if the positive charge and the almost of the mass of the atom
was located in a tiny central core.
- Rutherford proposed that the electrons were orbiting the central, massive
core like planets around the sun.
- Problems with model:
- Cannot explain atomic spectra - the model predicts that light will be
emitted in a continuous spectra.
- Predicts that atoms are unstable - electrons in circular orbits around
the nucleus would be centripetally accelerated. Accelerating electric charges
emit energy. The electrons' energy would decrease, causing the electrons to
spiral into the nucleus.
- Bohr model (1912)
- Bohr hypothesized that electrons moved around the nucleus in orbits, but
only certain orbits were allowed. Electrons in each orbit would contain a
definite amount of energy and could move in the orbit without radiating
energy.
- Light was emitted only when an electron jumped from one possible orbit
to another. When this occurs, a photon of energy is emitted or absorbed whose
energy was given by "hf".
- hf = Eupper - Elower
- Bohr proposed that allowed orbits have quantum numbers (n) of
1, 2, etx.
- The energy of a quantum level (n) of a hydrogen atom is given by:

- Problems with the Bohr model:
- Bohr assumed that electrons that move in a circle do not radiate energy
even though they are accelerating.
- Bohr's model could not explain how electrons moved from one energy level to
another.
- His model could not explain why there was a stable ground state or why
orbits were quantitized.
In 1923, Louis de Broglie proposed that matter also behaved like a wave. He
based his argument upon the symmetry of nature. If light acted like both a
wave and a particle, then matter should also act like a particle and like a
wave. He proposed that the wavelength of a material particle should be
related to its momentum just as the wavelength of a photon is related to
its momentum (p=h/l).
de Broglie wavelength is given by
l = h / m v
Quantum mechanics model of the atom:
- According to de Broglie's hypothesis, electrons have a wave nature, providing
an explanation for Bohr's model of the atom.
- de Broglie proposed that the electrons in orbit around the nucleus are
actually resonant standing waves.
- The electron can be envisioned as a circular standing wave that closed
on itself. If the wavelenght of the wave was such that the wave did not
close in upon itself, destructive intereference would occur.
- The only allowable standing waves contain a whole number of wavelengths.
Heisenberg Uncertainty Principle - a particle's momentum and position
cannot both be known precisely at the same time
Quantum Physics Sample Problems
Quantum Physics Homework



